No products in the cart.

ctDNA analysis based on NGS is a global trend
in early cancer detection.
ctDNA can be detected through a blood test and has been shown to be a valuable tool in the cancer early detection, especially for those healthy and asymptomatic.
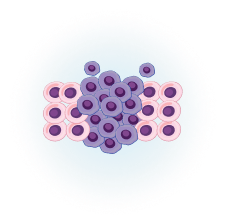
Cancerous cells are formed in the tumor
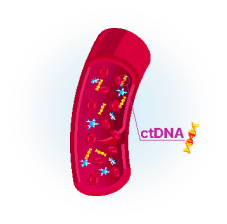
Tumor cells release DNA fragments to the bloodstream, called circulating tumor DNA (ctDNA)

Draw a blood sample (10ml) to extract cell-free DNA from plasma
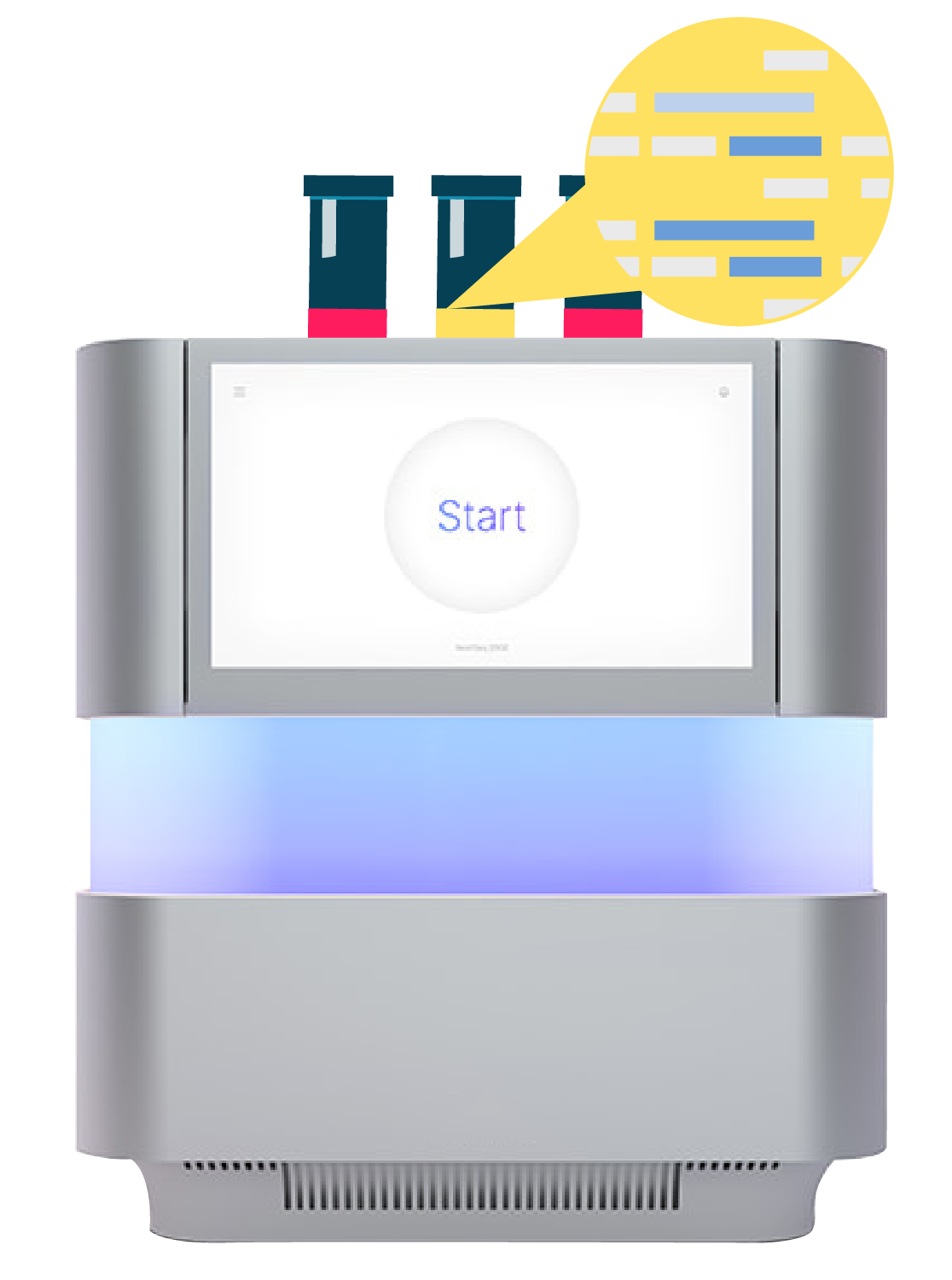
Apply next-generation sequencing to detect 5 different features of ctDNA from tumor cells compared with cfDNA from normal cell
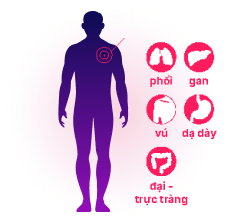
ctDNA is detected will be co-analysis with prediction of tumor origins in the scope of 5 cancers, based on advance machine learning data, to guide the further steps for doctors
How is SPOT-MAS different from hereditary cancer screening tests?
Hereditary cancer screening tests look for genes with mutations linked to cancer risks, and do not tell whether you have cancer at the time of testing.

ctDNA technology enables multi-cancer early detection (MCED) through a single blood draw. Non-invasive, convenient, accurate and accessible, the test is very promising in opening a new era in the world’s fight against cancer.

Cancer cells may develop so silently within our body for as long as 12 years without patients being aware of any symptom, until it is too late. Therefore, detecting cancer at early stages determines the efficacy of treatment.
of the most common types of cancer*
Proven accuracy
The licensed SPOT-MAS test is a supportive screening one, and does not replace current cancer screening guidelines.
X-ray & Mammogram
X-ray, CT-SCAN, MRI
Low-dose CT (imaging)
Colonoscopy
Gastroscopy
(*) Smoking more than 20 packs/year or passive smokers;
who drink more than 15 cans/week for men and eight cans/week for women;
people who are constantly exposed to toxic substances or a polluted environment.
(**) Family history of having been diagnosed with cancer.

The SPOT-MAS test is recommended for use in adults with elevated risk of cancer, such as those aged 40 years or older, those who carry genetic mutations, or those with unhealthy habits like smoking, drinking alcohol, getting hepatitis B, C. SPOT-MAS is not recommended for pregnant women or patients undergoing cancer treatment, or those with the history of bone marrow transplant, blood transfusion within 3 months.
The SPOT-MAS test is used to detect signals that suggest cancer through ctDNA released from cancer cells into the bloodstream and to predict the tumor origin of the cancer signal in the body. SPOT-MAS does not detect all cancers and not all cancers can be detected through ctDNA analysis. Therefore, SPOT-MAS should be used as recommended by the Doctor and as a supporting method for recommended routine cancer screening methods to help detect cancer EARLY.
It should be noted that SPOT-MAS is a screening test and SPOT-MAS results should be consulted by a healthcare expert, or a genetic specialist. The Doctor will interpret the results of the SPOT-MAS test based on information of your medical history, clinical symptoms, and other signals. A negative SPOT-MAS test result “no ctDNA signal detected” does not exclude all possibilities of having cancer, so it is still necessary for the routine check-up of cancer as recommended by a healthcare professional. With a positive SPOT-MAS test result “ctDNA signal detected” and the origin of cancer prediction, the Doctor will recommend follow-up diagnostic methods such as imaging, biopsy, etc. to confirm the presence of cancer. False positives and false negatives do occur.
When using SPOT-MAS tests for early cancer screening, some limitations of ctDNA analysis method released from cancer cells into peripheral blood should be kept in mind about the following results:
Source:
*According to the report of GLOBOCAN 2020, the 5 most common types of cancer in Vietnam include liver cancer (14.5%), lung cancer (14.4%), breast cancer (11.8%), %), stomach cancer (9.8%) and colorectal cancer (9%).
**According to Cancer Investigation (USA) , a research published by Gene Solutions in February 2023 on 2,975 people taking SPOT-MASTM cancer screening test. The test shows its sensitivity of 73.9%, specificity of 95.9%, positive predictive value (PPV) 58.1%, tumor tissue of origin (TOO) 84%
Copyright © 2020 GENE SOLUTIONS
Legal Representative: Nguyễn Hữu Nguyên
Enterprise No. 0314215140 – HCMC D.P.I issued on January 23, 2017
GET MEDICAL CONSULTATION FROM DOCTORS
Tư vấn di truyền